Study Guide
Field 043: Science—Chemistry
Sample Multiple-Choice Questions
Objective 0001
The Nature and Processes of Science (Standard 1)
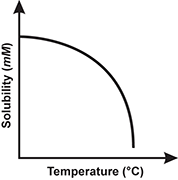
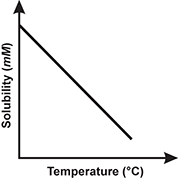
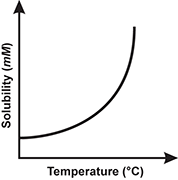
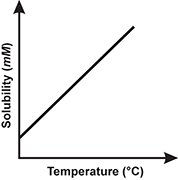
1. Use the table below to answer the question that follows.
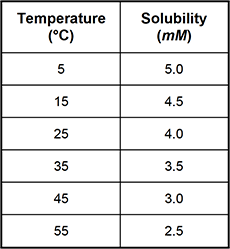
Which of the following line graphs best represents the data shown in the table?
- Answer
-
Correct Response: B.
This question requires the examinee to demonstrate knowledge of the analysis, interpretation, and communication of scientific data. The data in the table show that solubility decreases as temperature increases. This decrease in solubility is linear. The graph best representing this trend is the one with a straight line having a negative slope.
Objective 0002
Central Concepts and Connections in Science (Standard 2)
2. Cell theory is based on the fundamental tenets that all living organisms are made up of cells, cells are the fundamental unit of life, and cells arise from preexisting cells. When this theory was first proposed in the mid-1800s, it was met with skepticism. Which of the following statements best explains the source of this skepticism?
- Microscopes had not yet been invented.
- Many scientists of that time believed in spontaneous generation.
- The scientific method was not widely accepted at that time.
- Science was still deeply rooted in the principles of alchemy.
- Answer
-
Correct Response: B.
This question requires the examinee to demonstrate knowledge of the basic concepts and major principles of life science. Cell theory is widely accepted by scientists today. However, in the mid-1800s, many scientists still believed that living organisms formed spontaneously from nonliving matter. This belief was irreconcilable with the new idea that cells form only from preexisting cells. The conflict between these points of view led to skepticism of cell theory.
Objective 0003
Atomic Structure (Standard 3)
3. Which of the following pairs of ions is arranged in order of increasing ionic radius?
- Cl-, K+
- Fe2+, Fe3+
- O2-, S2-
- Mg2+, Al3+
- Answer
-
Correct Response: C.
This question requires the examinee to demonstrate knowledge of the relationship between subatomic particles and the organization of the periodic table. The size of an ion is determined by its nuclear charge, the total number of electrons, and the principal quantum number of the orbitals occupied by its valence electrons. For ions in the same group of the periodic table that carry the same charge, ionic radius increases from the top of the column to the bottom of the column. This is primarily due to differences in the principal quantum numbers of the valence electron orbitals. Of the ion pairs shown, O2- has a smaller ionic radius than S2-.
Objective 0003
Atomic Structure (Standard 3)
4. Use the equation below to answer the question that follows.
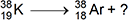
What is the missing product in the nuclear reaction shown?
- alpha particle
- positron
- beta particle
- gamma ray
- Answer
-
Correct Response: B.
This question requires the examinee to demonstrate knowledge of the types of emissions resulting from radioactive decay. In the nuclear equation shown, the atomic number decreases by 1 and the mass number remains unchanged. The emission of a positron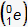 is consistent with these changes and it will lead to a balanced nuclear
equation.
is consistent with these changes and it will lead to a balanced nuclear
equation.
Objective 0004
The Properties of Matter (Standard 4)
5. According to kinetic molecular theory, particles in which of the following gas samples will display the greatest average speed at 300 K?
- 1 mol He
- 1 mol O2
- 1 mol Kr
- 1 mol Cl2
- Answer
-
Correct Response: A.
This question requires the examinee to demonstrate knowledge of the principles of kinetic molecular theory. While all gases at the same temperature have the same average kinetic energy, they all do not have the same average speed. The molar mass of a gas has a considerable effect on its root-mean-square speed (urms) as shown in the following equation where R is the ideal gas constant and T is temperature in kelvins: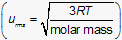 . While root-mean-square speed (urms) is not
the average speed, it is a close approximation of it, and it can be used to compare
the average speeds of a group of gases at the same temperature. As the equation
shows, the gas with the smallest molar mass will have the greatest root-mean-square
speed (urms). The molar mass of helium is less than the molar masses
of the other gas samples. As a result, it will have the greatest average speed at
300K.
. While root-mean-square speed (urms) is not
the average speed, it is a close approximation of it, and it can be used to compare
the average speeds of a group of gases at the same temperature. As the equation
shows, the gas with the smallest molar mass will have the greatest root-mean-square
speed (urms). The molar mass of helium is less than the molar masses
of the other gas samples. As a result, it will have the greatest average speed at
300K.
Objective 0004
The Properties of Matter (Standard 4)
6. The pressure of 2.00 mol of Xe in a 0.500 L sealed vessel at –5.00°C predicted by the ideal gas equation is nearly three times greater than the actual gas pressure in the vessel. Which of the following responses best explains this difference between theoretical and actual gas pressures?
- The volume of space occupied by the Xe atoms increases at low temperatures.
- The interactions between Xe atoms become more elastic at low temperatures.
- The speed at which the Xe atoms travel in the vessel increases at low temperatures.
- The attractive forces between Xe atoms become significant at low temperatures.
- Answer
-
Correct Response: D.
This question requires the examinee to demonstrate knowledge of the application of the gas laws to chemical systems. Pressures calculated using the ideal gas equation are based on the assumption that gas molecules do not interact with one another. This assumption does not always hold true at low temperatures. As the temperature decreases, the kinetic energy of the gas molecules decreases and the attractive forces between molecules become significant. As a result, colliding gas molecules do not bounce off one another with as much force as they do at higher temperatures. This leads to an observed pressure that is less than the pressure predicted by the ideal gas equation.
Objective 0005
Chemical Bonding (Standard 5)
7. The Lewis structure for BeCl2 vapor and the orbital diagrams for Be and Cl are shown below.
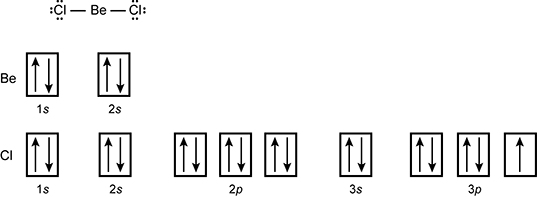
This information indicates that each Be–Cl bond in BeCl2 vapor is formed by the overlap of a 3p orbital from Cl and:
- a 2p orbital from Be.
- a 2s orbital from Be.
- an sp2 hybridized orbital from Be.
- an sp hybridized orbital from Be.
- Answer
-
Correct Response: D.
This question requires the examinee to demonstrate knowledge of the application of the valence-shell electron-pair repulsion (VSEPR) model and valence bond theory. The Lewis structure of BeCl2 shows that Be forms two equivalent covalent bonds with the Cl atoms. Each Cl atom has a partially filled 3p orbital that is involved in forming these bonds. However, the Be atom has a filled 2s orbital. In order to form two equivalent bonds with the Cl atoms, the 2s and a 2p orbital of Be hybridize to form two sp hybrid orbitals. Each covalent bond forms when a partially-filled 3p orbital of Cl overlaps with a partially-filled sp orbital of Be.
Objective 0005
Chemical Bonding (Standard 5)
8. Use the table below to answer the question that follows.
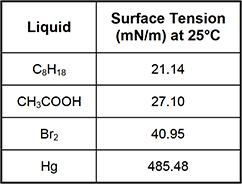
The surface tensions for four liquids at 25°C are shown in the table. The significantly greater surface tension of Hg can be attributed to which of the following phenomena?
- the lack of hydrogen bonding between atoms in liquid Hg
- the low atomic weight of Hg relative to the molecular weights of the other liquids
- the presence of metallic bonding between Hg atoms
- the compact shape of Hg atoms compared to those in the other liquids
- Answer
-
Correct Response: C.
This question requires the examinee to demonstrate knowledge of the relationship between intermolecular forces and the properties of matter. Molecules or atoms at the surface of a liquid are packed more closely together than molecules or atoms within the liquid. This is caused by forces pulling the surface molecules or atoms inward. The effect of this force is a reduction in the surface area of the liquid. Surface tension is a reflection of the strength of this inward force, and it specifically refers to the energy needed to increase the surface area by a specified unit of area. Substances with strong intermolecular attractive forces have a high surface tension. Mercury is a substance whose atoms are held together by strong metallic bonds. It is the presence of these bonds that causes mercury's surface tension to be significantly greater than those of the other substances listed in the table.
Objective 0006
Chemical Reactions (Standard 6)
9. Use the chemical equation below to answer the question that follows.
2NO(g) + 2Cl2(g) → 2NOCl(g)
Experimental data have revealed that the chemical reaction shown is first order in Cl2 and second order in NO. On the basis of this information, which of the following changes to a given set of experimental conditions will double the initial rate of the reaction?
- increasing the concentration of Cl2 by a factor of 2 and holding the NO concentration constant
- increasing the concentration of NO by a factor of 2 and holding the Cl2 constant
- increasing the concentrations of both Cl2 and NO by a factor of 2
- increasing the concentration of Cl2 by a factor of 2 and the concentration of NO by a factor of 4
- Answer
-
Correct Response: A.
This question requires the examinee to demonstrate knowledge of chemical kinetics, including reaction rates, rate constants, rate laws, and reaction order. Reaction order refers to the value of the exponents for the reactant concentrations in a rate law expression. On the basis of the information provided, the rate law expression for this reaction is rate = k[Cl2][NO]2. Given this rate law, the set of conditions that will lead to a doubling of the initial reaction rate is the one in which the concentration of Cl2 is doubled and the concentration of NO is held constant.
Objective 0006
Chemical Reactions (Standard 6)
10. Use the equation below to answer the question that follows.
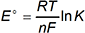
Which of the following types of questions can be answered using the equation shown?
- How many grams of product are produced in an electrolysis reaction?
- What is the length of time an electrochemical cell will operate before reaching equilibrium?
- How much electricity is needed to drive a nonspontaneous reaction?
- Are products present in greater amounts than reactants when an electrochemical cell reaches equilibrium?
- Answer
-
Correct Response: D.
This question requires the examinee to demonstrate knowledge of the principles and applications of electrochemistry, including electrolytic and galvanic cells, cell potentials, and cell equilibrium. The given equation relates the standard cell potential (E°) of an oxidation-reduction reaction to its equilibrium constant (K). The equation can be used to calculate the value of K for an oxidation-reduction reaction by substituting in values for E°, the ideal gas constant (R), temperature in kelvins (T), the number of electrons transferred (n), and Faraday's constant (F). The value of K provides information about the composition of the reaction mixture at equilibrium, such as whether the formation of products is favored over the formation of reactants.
Objective 0007
Thermochemistry (Standard 7)
11. Use the table below to answer the question that follows.
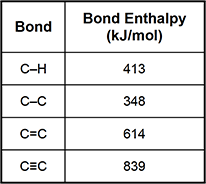
On the basis of the bond enthalpies shown in the table, which of the following combustible compounds possesses the greatest amount of potential energy?
- CH4
- HC≡CH
- C3H8
- H2C=CH2
- Answer
-
Correct Response: C.
This question requires the examinee to demonstrate knowledge of the energy changes associated with the formation and breaking of chemical bonds. Bond enthalpy values represent the average amount of energy needed to break a given type of bond in a gaseous molecule. Summing the bond enthalpy values for all of the bonds in a compound provides an estimate of the compound's potential energy. When total bond enthalpy values of equivalent molar amounts of each compound are compared, C3H8 is found to have the greatest amount of energy stored in its bonds.
Objective 0007
Thermochemistry (Standard 7)
12. A student adds 25.00 g of a pure metal at 85.00°C to a constant-pressure calorimeter containing 100.0 mL of H2O and records the change in H2O temperature. If the temperature of the H2O increases from 25.00°C to 26.55°C and there is negligible heat loss to the surroundings, what is the specific heat of this metal?
- 16.74 J/(g•K)
- 11.10 J/(g•K)
- 0.4438 J/(g•K)
- 0.3052 J/(g•K)
- Answer
-
Correct Response: C.
This question requires the examinee to demonstrate knowledge of the use of calorimetry to determine the amount of heat absorbed or released in chemical reactions and physical processes. The specific heat of the metal (s) can be calculated using the equation q = ms ΔT and solving for s. The heat change for the metal (q), the mass of the metal (m), and the change in temperature (ΔT) are known or can be calculated using the information provided. The heat change for the metal can be determined by calculating the heat change for the water as follows: q = 100.0 g H2O × ×
1.55K = 648.5 J. Since the heat absorbed by the water is equal to the heat lost
by the metal, the value of qmetal is –648.5 J. The temperature of the
metal changes from 85.00°C to 26.55°C for a difference of –58.45°C.
Since the Celsius scale and the Kelvin scale have units of equal value, a decrease
in temperature of 58.45°C is equal to a decrease in temperature of 58.45K. When
the values of q, m, and ΔT for the metal are substituted
into the equation q = msΔT, the specific heat of
the metal (s) is determined to be
×
1.55K = 648.5 J. Since the heat absorbed by the water is equal to the heat lost
by the metal, the value of qmetal is –648.5 J. The temperature of the
metal changes from 85.00°C to 26.55°C for a difference of –58.45°C.
Since the Celsius scale and the Kelvin scale have units of equal value, a decrease
in temperature of 58.45°C is equal to a decrease in temperature of 58.45K. When
the values of q, m, and ΔT for the metal are substituted
into the equation q = msΔT, the specific heat of
the metal (s) is determined to be 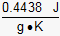 .
.
Objective 0008
Organic Chemistry and Biochemistry (Standard 8)
13. Which of the following characteristics of benzene can be attributed to the presence of pi bonds?
- its low solubility in water
- its rigid stable molecular structure
- its physical state at room temperature
- its greater density as a solid than as a liquid
- Answer
-
Correct Response: B.
This question requires the examinee to demonstrate knowledge of the composition, structure, and properties of organic compounds. The delocalized pi bonds in benzene are formed by the overlap of partially-filled unhybridized 2p orbitals on the six carbon atoms. In order for maximum orbital overlap to occur, the six carbon atoms need to be localized in the same plane. This orientation imparts a rigidity and stability to benzene's molecular structure.
Objective 0008
Organic Chemistry and Biochemistry (Standard 8)
14. When carbohydrates are not readily available, cells use other molecules as substrates for cellular respiration. Which of the following is an example of one of these alternative substrates?
- CO2
- RNA molecules
- H2O
- amino acids
- Answer
- Correct Response: D.This question requires the examinee to demonstrate knowledge of cellular respiration and major catabolic pathways. Glucose is the primary substrate used to produce energy in the process of cellular respiration. However, it is not the only substrate for this reaction. Amino acids can also serve as substrates for cellular respiration. The partial degradation of amino acids produces carbon compounds that can be used to form pyruvate, acetyl CoA, and other intermediates of glycolysis and the Krebs cycle.
Objective 0009
Science Instruction and Assessment (Standard 9)
15. Chemistry students are using molecular modeling software to support molecular geometry content presented in their textbook. Which of the following best explains how this software could be used to enhance students' understanding of this concept?
- Students are more likely to collaborate on problems involving challenging concepts when provided with software tools.
- The software provides students with a new way to test their knowledge of chemical nomenclature.
- Students consistently show a heightened interested in content that is presented in a digital format.
- The ability to represent molecules in three dimensions can help make an abstract concept more concrete.
- Answer
-
Correct Response: D.
This question requires the examinee to demonstrate knowledge of strategies and skills for using technological resources to enhance teaching and learning in science. Molecular modeling software can be used to produce three-dimensional representations of compounds. Students can use this software to compare the shapes of compounds having different molecular geometries. This type of technological resource can help make an abstract concept such as molecular geometry more concrete.